1. What are Polyclonal & Monoclonal antibodies?
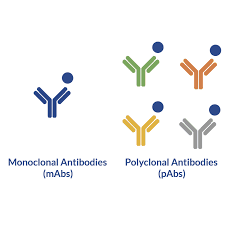
Antibodies (Ab) are immunoglobulins produced against the antigen (An) by activated B -Lymphocytes. On activation, B cells differentiate into plasma cells and memory cells. Plasma cells proliferate to form clone of cells and produce large number of antibodies targeted against the antigen that had stimulated the entire process. However, Ans have multiple antigenic determinants or epitopes. Hence, on encountering an An, multiple clones of B cells are generated which will result in the production of antisera containing Abs or Igs of different classes with specificities against different epitopes of the same An. Such Abs are called “Polyclonal Abs”.
Abs
produced by clones of a single B lymphocytes and directed against single
antigenic determinant or epitopes are called “Monoclonal Abs”. Means,
these Abs will bind with single specific epitope on an An. Ie; they are
identical Abs with same specificity. Polyclonal Abs are heterogenous population
of Igs whereas Monoclonal Abs are population of single type of Ab. Monoclonal
Abs are useful tools for diagnostic and research techniques. The technique used
for the production of large amount of monoclonal Abs is called Hybridoma
technology.
2. What is Hybridoma technology?
Hybridoma
technology is a versatile and efficient method for large scale production of
monoclonal antibodies against desired antigen. Hybridomas- are somatic
cell hybrids produced by fusing Ab producing B lymphocytes from spleen cells
and Myeloma cells. Production of desired monoclonal Abs from these hybridoma
cells is called hybridoma technology. This method was developed by Georges J F
Kohler and Cesar Milstein in 1975 and jointly awarded Nobel prize in Physiology
& Medicine in 1984 for this work.
Step 1- Immunization of laboratory animal
Lab
animal like Swiss albino mice is immunized with the An against which we have to
produce Abs. This An will be having multiple epitopes on it. B lymphocytes get
activated against the epitopes of the An. After few weeks, the mice is
sacrificed and spleen is removed aseptically.
Spleen is the secondary lymphoid organ and we can easily harvest B
lymphocytes from spleen. Spleen is subjected to mechanical and enzymatic disruption
followed by density gradient centrifugation to separate activated B cells from
normal B cells of spleen. Now we have B cells capable of producing Abs against
the multiple epitopes of the injected An.
Step 2- Fusion of cells to produce Hybridomas
B
cells have only short life span in cell culture. Hence, to increase their life
span in laboratory conditions they are fused with Myeloma (Blood cancer) cells.
Myeloma cells used here are mutated Myeloma cells. Myeloma cells are cancerous
B cells (Plasma cells) that can divide indefinitely in culture media. Their 2
genes are mutated. One is HGPRT (Hypoxanthine phosphoribosyl transferase) gene.
Hence, they are not able to synthesize nucleotides by the Salvage pathway. Second
mutated gene is Ig gene. As a result of this mutation myeloma cells could not
synthesize their own Abs. These
mutations are represented as HGPRT- & Ig-.
Activated
B cells and Myeloma cells are fused in the presence of chemical fusogen Polyethylene Glycol (PEG). As a result of cell fusion, 5 types of cells
are obtained.
- · Unfused
B cells
- · Fused
B cells
- · Unfused
myeloma cells
- · Fused
myeloma cells
- · Hybrid
cells (Hybridomas)
Hybridomas
are formed by the fusion of B cells and myeloma cells. Hybridomas will be of
different types depending on the type of B cell fused with myeloma cells. Next task
is the selection of this hybridomas from mixture of above cells.
Step 3- Selection of Hybridomas using HAT medium
H=
Hypoxanthine
A=
Aminopterin
T=
Thymidine
HAT
medium is a selection medium for mammalian cell cultures. During cell
multiplication, nucleic acid synthesis takes place in 2 pathways. The De
novo pathway & Salvage pathway. Aminopterin in HAT medium blocks the De
novo pathway. Hence the only the cells that can synthesize their nucleic
acid using Salvage pathway can survive in HAT medium. H & T are major metabolites
in Salvage pathway and HGPRT is the key enzyme. So HGPRT- cells die
in HAT medium.
Once
the hybridoma mixture is added to the HAT medium, fused and unfused B cells die
out in few days as they have short life span in culture media. They can not
divide indefinitely in cell cultures. Fused and unfused myeloma cells will also
die as they are HGPRT- and Aminopterin blocks De novo pathway
in HAT medium. Hybrid cells survive in HAT medium as they are HGPRT+ due
to the activated B cell and divide indefinitely, property due to the myeloma
cell. Hence, what will be remaining in HAT medium will be hybrid cells that are
able to produce Abs against the different epitopes of the particular An. Now these
hybridomas are mixture of B cells producing Abs of different specificities. Now
our aim is to select and propagate hybrid cells that produce single type of Ab.
So, we have to separate these hybridomas and grow them individually. So next
step is isolation of hybridomas of single specificity.
Step 4 - Isolation of Hybridomas of single specificity
Isolation
of hybridomas of single specificity is achieved by limiting dilution method. In
this technique cells are distributed at very low density in multi well culture
plates so that each well contain a single cell only.
Step 5 –
Screening of Abs produced
Next
step is the screening of Abs produced by individual hybridoma cells in the multi
well culture plates. Collected supernatants could be analyzed for Abs by
techniques like ELISA and RIA.
Step 6 – Cloning & Propagation
Once
the hybridomas producing the desired Abs are identified they are isolated, cloned
and propagated. Now we have hybridomas producing Abs of single specificity or
monoclonal Abs.
Step 7 – Characterization and storage
Finally,
mAbs are characterized and stored, usually in liquid nitrogen. Now they can be
readily used in treating and diagnosing diseases.
3. Advantages & applications of Hybridoma technology
Discovery
of hybridoma technology was a revolution in immunology.
- · Hybrid
cells can be maintained indefinitely in cultures for the continuous production
of mAbs in vitro.
- · Invivo
maintenance can be done by injecting hybrid cells in the intraperitoneal cavity
of mice and mAbs can be harvested from the ascitic fluid produced.
- · Hybrid
cells can be kept frozen for prolonged usage.
- · Powerful
tool of passive immunization.
- · Numerous
therapeutic, diagnostic (bacterial, viral & other Ans) and research
applications.
- ·mAbs
against various Ans are now used in commercially available immunofluorescence &
ELISA kits.



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.